Pandemics such as COVID-19, Ebola, and the 2009 influenza instilled the need for a well-equipped domestic vaccine manufacturing industry in the minds of African leaders. Currently, due to insufficient local production, the continent depends heavily on imports from other countries, with the imports satisfying about 99% of vaccine demand in the continent. However, thanks to recent significant FDI, the vaccine industry in Africa had a market potential of around US$1.3 billion as of 2021 and is expected to range between US$2.3 billion and US$5.4 billion by 2030, as per McKinsey estimates.
Vaccine sovereignty is the need of the hour for the African continent
One of the most important lessons the COVID-19 pandemic has given to Africa is the pressing need to ramp up vaccine production locally. Biotech firms, such as Moderna and Pfizer, developed COVID-19 vaccines faster than any other producers. However, these vaccines were not easily accessible to most African countries.
Africans, in general, lack access to affordable and quality healthcare. Preventable diseases, such as pneumonia, malaria, and typhoid fever, have high fatality rates across the continent. This calls for localized production of pharmaceuticals and vaccines to lower the economic burden of these diseases and facilitate better access to affordable healthcare.
Currently, Africa relies heavily on other countries, such as China and India, for its pharmaceutical needs. The paucity of localized pharma production aggravates healthcare and vaccine inequity across the continent. To substantiate this, the COVID-19 vaccination rate at the beginning of 2022 in 16 African countries was less than 5% on average.
Currently, Africa consumes around 25% of the global vaccine production, whereas it produces less than 1% of its vaccine needs locally, as per the African Union (AU). Therefore, a lot remains to be done to materialize the goal of achieving 60% of vaccine needs to be satisfied locally by 2040, the vision of the Partnerships for African Vaccine Manufacturing (PAVM) under Africa CDC.
Increasing the vaccine production capacity from 1% to 60% in 15-16 years is not an easy task. Considering this, PAVM designed a continental plan for creating a vaccine production ecosystem capable of achieving the 60% target. This plan, called the PAVM Framework for Action (PAVM FFA), assessed that the African vaccine manufacturing industry would be expected to have increased the number of their vaccine production factories from 13 in 2023 to 23 (11 form, fill, finish, or F&F factories and 12 end-to-end factories) by 2040 providing a total of 22 priority products by 2040. It will require dedicated efforts from all involved stakeholders, such as producers, biopharma companies, industry associations, regulatory bodies, and academia.
Significant FDI will aid in driving localized vaccine production in Africa
The continent is attracting considerable FDI from the USA and Europe for vaccine development. Several foreign biotechnology firms are partnering with African governments to venture into localized vaccine production.
In March 2023, US-based biotechnology company Moderna partnered with the Kenyan government to set up a production facility for making messenger RNA (mRNA). The proposed annual capacity of Moderna’s first-ever facility in Africa is around 500 million doses of vaccines. The facility is expected to produce drug substances or active pharmaceutical ingredients and the final product for the entire continent.
In another example, a Germany-based biotechnology company, BioNTech, is contemplating commencing production of mRNA-based vaccines in its Rwanda facility in 2025. The construction of the facility began in 2022. With an investment of around US$150 million, this is Africa’s first mRNA manufacturing facility built by a foreign company. The proposed annual capacity of BioNTech’s mRNA facility is about 50 million vaccine doses. BioNTech also plans to set up mRNA factories in other African countries, such as South Africa and Senegal, and plans to produce vaccines for malaria, tuberculosis, HSV-2, and HIV in the future.
In September 2023, the South African government partnered with the KfW Development Bank of Germany. As per the agreement, South Africa will receive €20 million from Germany’s KfW Development Bank over five years for developing and manufacturing mRNA vaccines. The fund will be utilized for equipment procurement and API certification for vaccine production in South Africa.
A consortium of the Global Alliance for Vaccines and Immunizations (GAVI), AU, and Africa CDC established the African Vaccine Manufacturing Accelerator (AVMA) with the intent of fostering a sustainable vaccine industry. The formation of AVMA involved donors, partners, industry stakeholders, and non-governmental and not-for-profit organizations. GAVI planned to expand its supplier base, mainly in Africa, in 2021. Furthermore, the global alliance announced the commencement of around 30 vaccine manufacturing projects across 14 African countries.
Moreover, as of December 2023, over US$1.8 billion is planned for investment by a collaboration between the French government, Africa CDC, and other European and international investors to streamline the development and production of vaccines across the continent.
Desire to ensure vaccine effectiveness is seen as a biased vaccine preference
African governments are not only proactively putting in dedicated efforts to attract considerable FDI to build and strengthen the continent’s vaccine manufacturing industry, but they also focus on good quality, effective vaccine types. However, some perceive this as a lack of interest from the African governments to buy non-mRNA vaccines made by local companies.
For example, Aspen Pharmacare, a South Africa-based biotechnology company, put significant investments in ramping up the capacity of its manufacturing facility to produce viral vector vaccines against COVID-19. The company announced in November 2020 that it would be formulating, filling, and packaging the COVID-19 vector vaccine made by J&J. It also received €1.56 million investment from Belgian investors, BIO, the Belgian Investment Company for Developing Countries, which is a JV between the European Investment Bank (EIB) and several European DFIs.
However, millions of J&J COVID-19 vaccine doses made in South Africa were exported to Europe by J&J without the knowledge of the South African government, to support Europe’s domestic vaccine demand in August 2021, not complying with the initial agreement of vaccine distribution within the African continent. This created a political impasse between European and African governments over the distribution of the vaccines, which, in turn, delayed their production as the standoff resulted in a long waiting time for Aspen Pharmacare to produce the COVID-19 vaccine.
Ultimately, by September 2021, the European countries agreed to return 90% of the J&J vaccines to Africa. In March 2022, J&J gave Aspen Pharmacare the license to manufacture and distribute the vaccine under its brand name, Aspenovax. The expected production capacity of Aspenovax was around 400 million doses. However, not a single order came from African governments.
According to Health Policy Watch News, the reason for this was the rising production of Pfizer and Moderna’s mRNA COVID-19 vaccine distributed by COVAX that was being opted for by most African governments. Thus, in August 2022, Aspen Pharmacare had to close its production line, stating non-existent demand in Africa, partly due to the subsidence of the pandemic and partly due to African governments’ lack of interest in non-mRNA vaccines. The company could not sell a single dose of the vaccine, owing to multiple factors, starting from what was perceived as the lack of government’s intent to purchase home-grown vaccines to delayed production due to the Europe-Africa political clash and the rising inclination of the world towards mRNA vaccines.
It is interesting to note that of the total Covid-19 vaccines Africa administered to its residents, 36% were J&J vector vaccines, shipped directly from the USA.
Technology transfer hub and know-how development initiatives are set
To strengthen vaccine production capacity in low- and middle-income countries (LMICs), the WHO declared the establishment of a technology transfer hub in Cape Town, South Africa, in June 2021. In February 2022, WHO said that Nigeria, Kenya, Senegal, Tunisia, and South Africa will be among the first African countries to get the necessary technical expertise and training from the technology transfer hub to make mRNA vaccines in Africa.
Afrigen Biologics, a South Africa-based biotech firm, is leading this initiative. As Moderna did not enforce patents on its mRNA COVID-19 vaccine, Afrigen Biologics could successfully reproduce the former company’s vaccine, capitalizing on the data available in the public domain. As per an article published in October 2023, Afrigen Biologics reached a stage where its vaccine production capabilities are appropriate for “phase 1/2 clinical trial material production”. Additionally, in collaboration with a Denmark-based biotech firm, Evaxion, Afrigen is developing a new mRNA gonorrhea vaccine.
Besides setting up a technology transfer hub in South Africa, academic institutions are partnering with non-profits as well as companies to reinforce the development of necessary technical know-how and training required for vaccine manufacturing. One such example is the development of vaccines in Africa under the partnership of Dakar, Senegal-based Pasteur Institute (IPD), and Mastercard Foundation. Approved in June 2023, the goal of MADIBA (Manufacturing in Africa for Disease Immunization and Building Autonomy) includes improving biomanufacturing in the continent by training a dedicated staff for MADIBA and other vaccine producers from Africa, partnering with African universities, and fostering science education amongst African students.
Read our related Perspective:
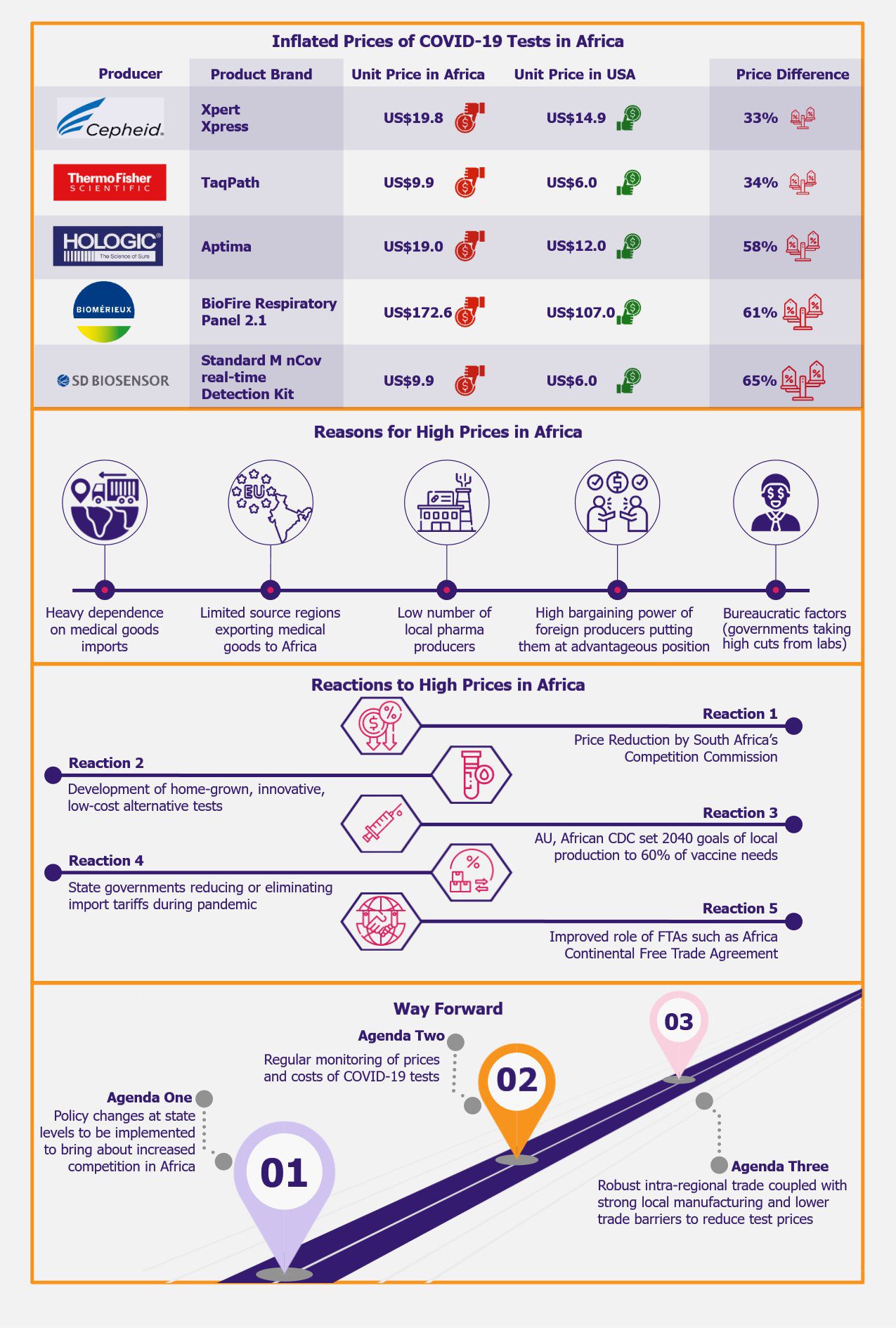
Inflated COVID-19 Tests Prices in Africa
Although significant initiatives are underway, challenges exist
With 13 vaccine manufacturing companies and academic organizations across eight African countries, the continent’s vaccine industry is in its infancy. However, the current vaccine manufacturing landscape includes a mix of facilities with capabilities in F&F (10 facilities), R&D (3 facilities), and drug substance (DS) or active pharmaceutical ingredients (API) development (5 facilities).
One of the challenges African vaccine producers face is not being able to become profitable in the long run. In 2023, a global consulting firm, BCG, in collaboration with BioVac, a South Africa-based biopharmaceutical company, and Wellcome, a UK-based charitable trust that focuses on research in the healthcare sector, conducted a detailed survey exploring stakeholder perspectives on challenges and feasible solutions. The respondent pool consisted of a diverse set of stakeholders spanning across Africa (43%), LMICs (11%), and global (46%). A total of 63 respondents from various backgrounds, such as manufacturers, industry associations, health organizations, regulators, and academic organizations, were interviewed across the regions above. According to this research, most vaccine producers in Africa who were interviewed said that profitability is one of their key concerns. This leads to a lack of foreign investments required for scaling up, which in turn creates insufficient production capacity, thereby increasing the prices of vaccines. Therefore, these producers are unable to meet considerable demand for their products, and their business model becomes unsustainable.
Continued commitment and support from all stakeholders are necessary for achieving a sustainable business model for vaccine producers in Africa and, consequently, for the industry at large. However, it has been observed that the support from global, continental, and national levels of governments and other non-government stakeholders, such as investors, donors, partners, etc., tend to diminish with the declining rampage caused by epidemics in Africa. Therefore, this poses a severe challenge to strengthening the vaccine production industry in Africa.
In another 2023 study, by a collaboration between the African CDC, the Clinton Health Access Initiative (CHAI), a global non-profit health organization, and PATH, formerly known as the Program for Appropriate Technology in Health, involving 19 vaccine manufacturers in Africa, it was suggested that the current vaccine production capacity including current orders to form/fill/ finish using imported antigens is nearly 2 billion doses. In contrast, the current average vaccine demand is 1.3 billion doses annually. In addition, there is a proposed F&F capacity of over 2 billion doses. Thus, if Africa can materialize both current and proposed plans of producing F&F capacity vaccines from imported antigens, the study concludes that the continent will reach a capacity of more than double the forecasted vaccine demand in 2030. Overcapacity will lead to losses due to wastage. Thus, not all vaccine producers will be profitable in the long term. This may challenge the African vaccine manufacturing industry to be profitable.
Moreover, Africa’s current domestic antigen production capacity is lower than what is required to meet PAVM’s vaccine production target of 60% by 2040. In addition, a large part of the existing antigen capacity is being utilized to make non-vaccine products. Although antigen production plans are underway, these will not suffice to narrow the gap between demand and production of antigens domestically in Africa.
EOS Perspective
To create a local, financially sustainable vaccine manufacturing industry with output adequate to support the continent’s needs, it is necessary to create an environment in which producers can achieve profitability.
Initiatives such as technology transfers and funding will only be fruitful when their on-the-ground implementation is successful. This will require the involvement of all stakeholders, from the state governments to bodies that approve the market entry of vaccines. All stakeholders need to be steadfast in their actions to achieve the ambitious target of 60% of vaccine needs to be met from local production by 2040 without compromising on the accuracy and quality of the vaccines.
One of the most vital aspects of the necessary planning is for stakeholders to ensure that even after the pandemic and its aftermath are entirely gone, the effort towards establishing facilities, creating know-how, and training a workforce skilled in vaccine development and production does not stop.
The focus should extend beyond COVID-19, as there are many other preventable diseases in Africa, such as malaria, pneumonia, tuberculosis, and STDs, against which vaccines are not yet produced locally. These areas provide a great opportunity for vaccine producers and associated stakeholders to continue being interested and involved in vaccine production and development in Africa.