Peer-to-peer (P2P) lending has emerged as a global financial phenomenon. It has revolutionized the way individuals access loans. The innovation of P2P lending has experienced varying degrees of success and turbulence in different regions, notably India, China, and the USA. Understanding the reasons behind the rise and fall of P2P lending across these major markets provides critical insights into the global dynamics of this industry.
P2P lending – good old loans with a modern take
Peer-to-peer (P2P) lending is giving loans through an online platform that connects lenders and borrowers to exchange goods, services, or money directly by eliminating traditional intermediaries such as banks. Financial technology facilitates P2P lending, directly connecting individuals or businesses with investors.
Lenders and borrowers need to register with a P2P platform before conducting any transactions. The registration entails an AI-based evaluation of the borrowers to assess their credit score, employment details, income, and credit history. It also monitors their social media activities, including usage patterns and interactions. Using these assessments, the borrowers’ creditworthiness is determined, categorizing them into various risk tiers and informing the interest rates offered.
Subsequently, lenders can make informed decisions about lending money based on borrowers’ assessed scores. This empowers them to select suitable borrowers and enables borrowers to choose appropriate lenders. The P2P platform charges fees from both parties for its services instead of deriving profit from monthly installments.
To mitigate fraudulent activities, certain regulatory bodies oversee these platforms to ensure compliance with regulations and maintain transparency. For example, P2P lending in the USA is regulated at the federal and state levels. The US Securities and Exchange Commission (SEC) oversees the investors of the P2P lending platforms, while the Federal Trade Commission and the Consumer Protection Financial Bureau oversee the borrowers. In India, all P2P lending platforms must register as Non-Banking Financial Companies (NBFC)-P2P Lenders with the Reserve Bank of India (RBI).
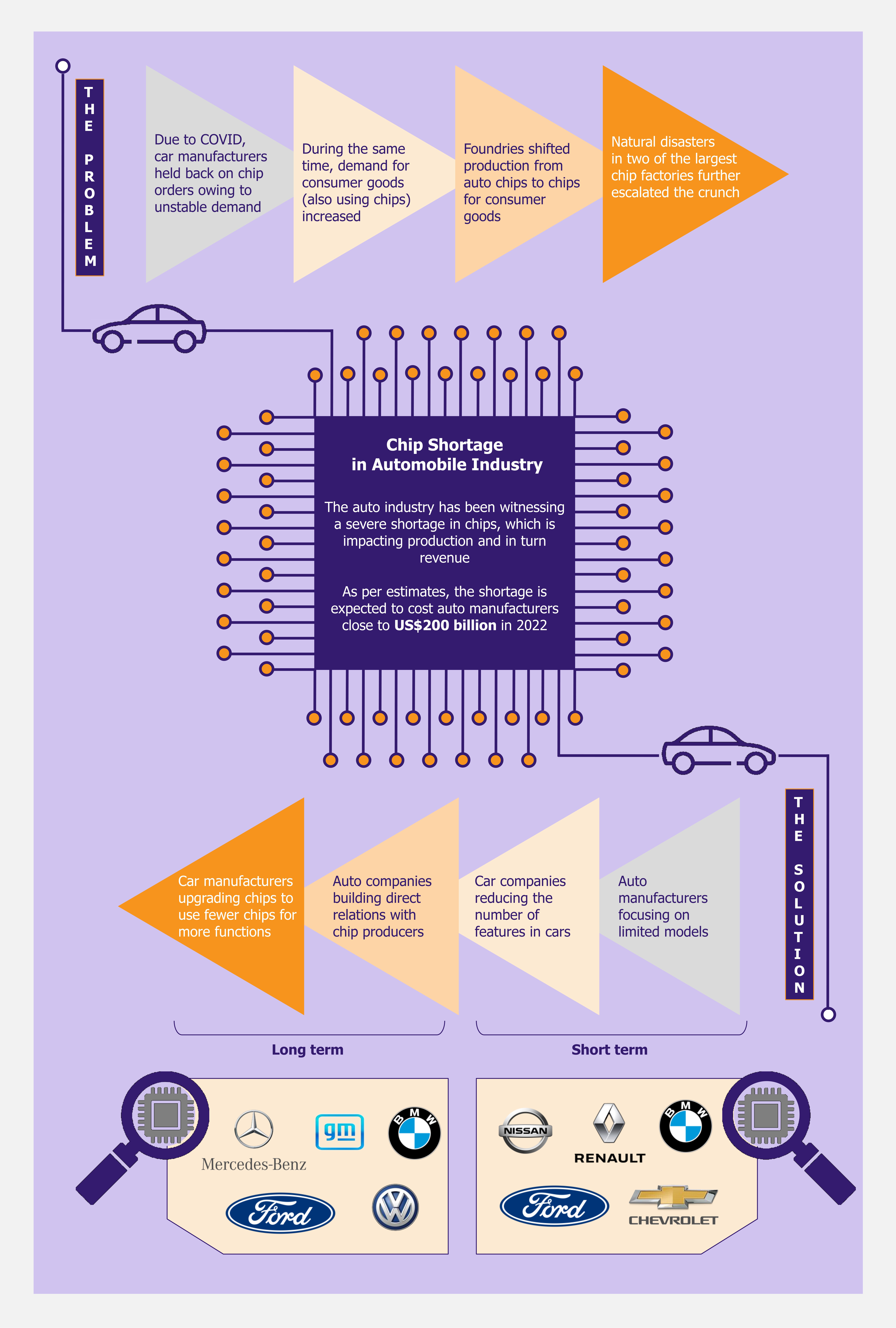
The global P2P lending market is expected to reach US$705.81 billion by 2030 up from US$83.79 billion in 2021, at a 26.7% CAGR during 2022-2030, according to Precedence Research.
In addition to the increasing demand for financial services, factors such as lower operating fees compared to traditional financial services, quicker loan approvals, and the adoption of digitization in the banking sector drive the growth of the P2P lending market.
China’s P2P lending – started strong but faced a downturn
China’s P2P lending industry witnessed speedy development since 2007. There were 3,383 P2P lending platforms running in China with around RMB 130 billion (~US$18.2 billion) in combined monthly transactions in January 2016, as per the Home of Online Lending, an organization that collects and assembles P2P data from various sources in China. Founded in August 2007, PPDai or Paipaidai, currently known as FinVolution Group, was the first online P2P lending platform in China. PPDai was listed on the New York Stock Exchange in November 2017.
However, this burgeoning growth of the P2P lending industry in China was unsustainable and short-lived. This was evident from the fact that out of 6,607 P2P lending platforms, 6,277 were closed and problematic, leaving only 330 P2P lending platforms in business in China as of August 2020, as per the Home of Online Lending. As of August 2020, the lenders of the collapsed P2P lending industry of China owed depositors US$115 billion.
There were several Ponzi schemes related to untrustworthy P2P lending platforms enticing potential investors with attractive bonuses for referring family and friends, as reported by the Chinese media by the end of 2015. For example, in early 2015, Ezubao, with 900,000 investors, went bust when it turned out to be a Ponzi scheme with US$9 billion. Some P2P platforms were found creating fictional information about the borrowers in order to create groups of assets, and these platforms utilized funds to fulfill their own business requirements.
Although until early 2016, no regulatory authorities were overseeing P2P platforms in the country, it was believed that the Chinese government was observing the industry closely. Three bodies (The China Banking Regulatory Commission regulating P2P lending business, the Central Ministry of Industry and Information Technology supervising the telecom business of P2P lending, and the Cyber Administration of China developing rules, managing administrative licenses, and control over internet regulation and censorship in China) together announced the Interim Measures on Administration of the Business Activities of Peer-to-Peer Lending Information Intermediaries (“Interim Measures”) in August 2016.
Interim Measures became China’s first regulatory framework for the P2P lending industry. According to the Measures, a P2P lending platform’s scope of business in China is limited to acting as lending information intermediaries. As per the new rules, P2P lending platforms were mandated to establish custodian accounts with registered financial institutions for investor and borrower funds previously held by them. This was done to decrease the risks associated with situations when P2P lending platform owners flee with the investors’ money.
Interim Measures also mandated that P2P lending platforms register with the local financial regulatory body. The Measures provided P2P lending platform owners with a twelve-month timeline for implementing all the mandates. However, there was a delay in the implementation, as the registration and rectification processes were scheduled to be completed by June 2018, but they were not complete as of August 2018.
The exponential growth of P2P lending platforms in China resulted in several crashes due to cash shortfalls, defaults, frauds, and closures, causing massive financial losses for lenders. Such market scandals made it difficult for investors and borrowers in China to survive. They presented difficulties in acquiring financial resources, and the platforms faced a situation where investors started withdrawing their investments, thus bringing about the ultimate crash of the P2P lending industry in China.
Indian P2P lending – bright future fueled by regulators
P2P lending started in early 2014 in India. However, it began gaining significance in September 2017 when the Reserve Bank of India (RBI) decided to regulate P2P lending in the country.
People in India started using online platforms to borrow and lend funds to various untapped markets characterized by less developed infrastructure and lower investment activity. The method of borrowing changed over time. Borrowers who found it difficult to access credit from financial institutions were borrowing money from relatives, friends, acquaintances, lenders, colleagues, and business partners. The revolution took place via the intervention of digital ways of funding the credit ecosystem.
In September 2017, RBI introduced regulatory guidelines that ensured P2P lending through non-banking financial companies (NBFCs). In October 2017, RBI published a different framework for the P2P lending platforms. RBI categorized these rules as NBFC-P2P. The regulatory norms have enabled P2P lending platforms to create adaptable lending and borrowing models, including the development of flexible loan tenures, interest rate structures, and more.
Later, in 2018, RBI published a list comprising names of the first five companies registered with NBFC-P2P lending. The registration list helped ensure a secure, regulated sector and protect the interests of lenders and borrowers. RBI, in one of its regulations, mentioned a cap of Rs.5,000,000 (~US$60,000), which means if lenders invest money above Rs.1,000,000 (~US$12,000) across P2P platforms, they are required to submit a certificate from a practicing Chartered Accountant certifying a minimum net worth of Rs.5,000,000. This also means that the borrower must certify the difference between their assets and liabilities to show their financial strength. The introduction of the cap discouraged many lenders from giving out big loans.
According to RBI, fund transfers between participants on the P2P lending platform should be made through the escrow account mechanism. This means that all transactions will be processed via bank accounts, and cash transactions are strictly prohibited.
RBI mandated that P2P lending platforms be members of the Credit Information Companies, entities that maintain credit-related information about businesses and individuals. This regulation by RBI was welcomed by P2P platforms but separated less powerful players from the P2P market. The inclusion of rules has brought higher transparency, credibility, and stability to P2P lending. However, they have also increased the operation cost for P2P lending platforms and decreased the activities of lenders and borrowers.
All these changes have helped borrowers obtain loans more easily and protected lenders from fraudulent activities. According to IndustryARC, India’s P2P lending market is predicted to reach US$10.5 billion with a CAGR of 21.6% between 2021 and 2026. Market transparency in P2P lending, facilitated by technologies such as blockchain and smart contracts, has contributed to the growth of the P2P lending market.
Government promotion of cashless technology in P2P lending has reshaped the financial sector, gaining significant momentum over the past years. The introduction of AI and machine learning, along with RBI norms, has created a more secure marketplace for investors and borrowers. Innovations and new players in the P2P market are expected to impact the growth of P2P lending in the future.
P2P lending in the USA – star performer driven by technologies
The P2P lending market shows significant growth in the North American market with a larger size, higher revenue, and rapid growth. Several platforms, such as Lending Club (founded in 2006) and Prosper (founded in 2005), supported the growth of the P2P lending market in the USA by making P2P lending easy and secure. These platforms helped in attracting a large number of borrowers and investors. In the USA, the adoption of mobile and digital technologies such as Venmo, which was acquired by PayPal in 2013, and Squash Cash increased customer interest in digital transfer capabilities.
The USA has achieved remarkable success in P2P lending compared to other countries, partially due to the implementation of various payment technologies, including the EMV (Europay, Mastercard, and Visa) smart payment card protocol used as an electronic payment method. This success can be attributed to the presence of adequate legal frameworks and well-defined strategies for generating revenue.
One contributing factor to the rise of P2P lending in the USA has been the emergence and growth of small and medium-sized enterprises (SMEs actively involved in P2P lending activities). These platforms helped reduce the cost of office setups, maintenance, staffing, etc., and thus helped boost the growth of P2P lending.
One of the reasons behind the increase in the P2P lending market was the COVID-19 pandemic in 2020. At a time when major businesses and organizations were facing difficulties regarding finance and operations, P2P lending platforms helped them to raise funds for their operations through online lending platforms such as i2iFunding, Faircent, Lendbox, etc., allowing a direct lending process without the involvement of third-party participants, such as banks.
Technological advancements, such as blockchain, are another reason behind the increase in the P2P lending market in the USA. They eliminated the need for physical branches and reduced operational costs. They reached global audiences such as individuals and businesses in underserved or remote areas. They also helped in reducing the risk of fraud and improve financial transactions. Undoubtedly, the P2P lending market is growing largely thanks to the adoption of new technologies.
EOS Perspective
Peer-to-peer (P2P) lending has shown distinct trends in India, China, and the USA. India and China witnessed a decline in their P2P lending markets due to regulatory hurdles aimed at addressing issues such as fraud and investor protection. Conversely, the USA experienced a surge in P2P lending activities. This uptick can be attributed to a well-established regulatory framework and a sustained appetite for alternative lending solutions. P2P lending platforms in the USA have been able to offer borrowers access to credit while providing appealing investment opportunities to lenders, all while adhering to regulatory standards.
Many new developments in P2P lending are helping the platforms become successful. One such development is the integration of decentralized finance (DeFi), a financial technology that works on a secure distributed ledger. The DeFi technology, born in 2018, aims to create a transparent, open, and permissionless financial system operating on blockchain networks such as Bitcoin or Ethereum.
DeFi in the USA empowers individuals with P2P digital exchange by challenging the centralized financial system by eliminating banks’ fees and other charges. DeFi allows a P2P lending platform to access a global pool of liquidity (which means a collection of digital assets to enable trading on DeFi), reduces costs and risks, and offers more flexible and customized products.
Artificial Intelligence and Machine Learning will continue to be the solutions that transform P2P lending with better data analysis, credit scoring, risk assessments, and fraud detection capabilities. AI will also allow for efficient and more personalized services to both lenders and borrowers.
Regulatory authorities, with their frameworks, have saved several platforms from data breaches, tax compliance issues, consumer protection concerns, and cyberattacks. These authorities, together with industrial associations, will continue to create innovative and adaptive solutions such as sandbox programs (a time-bound, controlled, and live testing environment involving parameters within which the firm must operate).
Looking at the history of some of the key P2P lending markets, it is evident that creating a more robust, secure, and dependable P2P lending ecosystem necessitates technological innovations and establishing a practical regulatory framework to ensure the safety of financial activities.