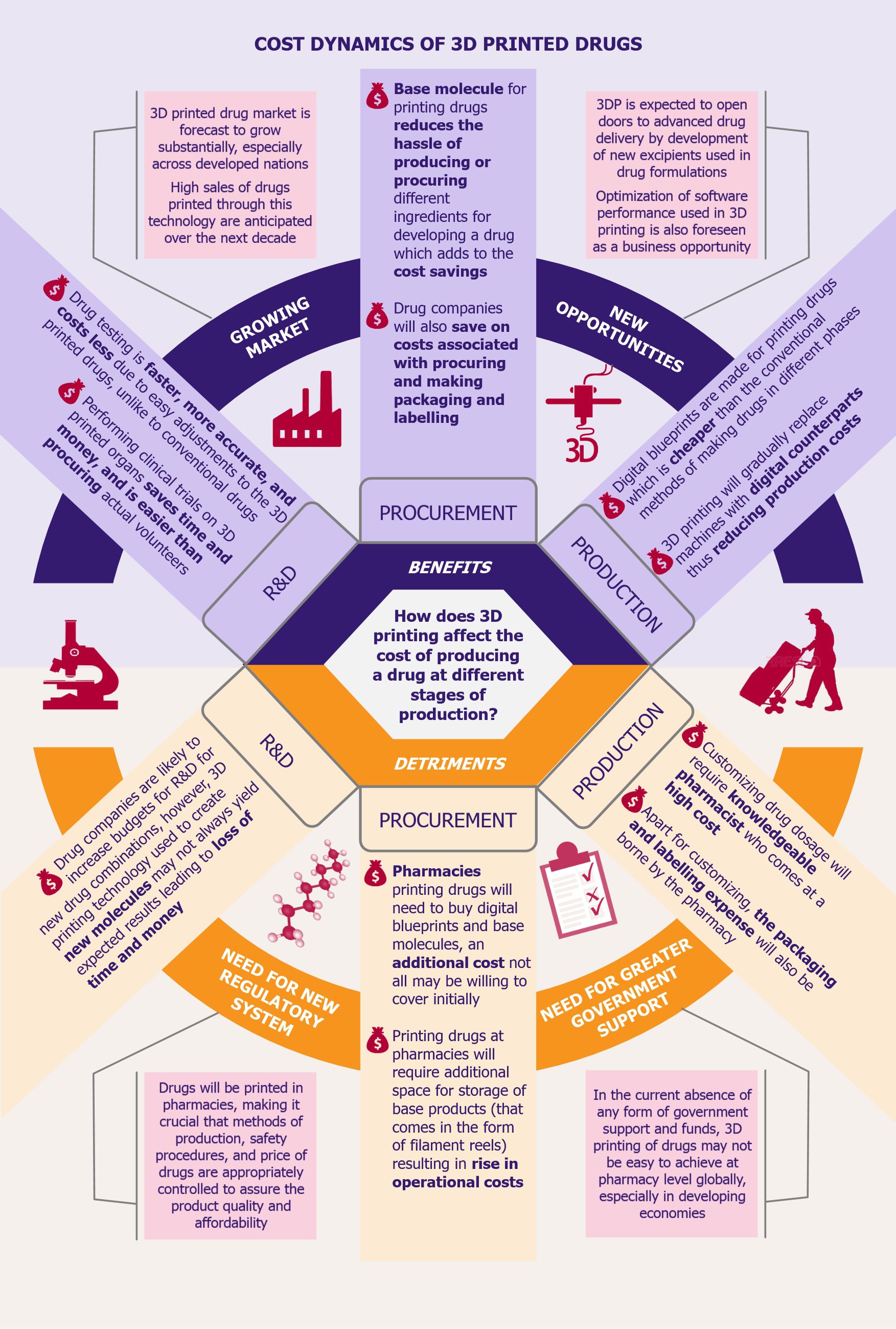
Medical industry needs no introduction to 3D printing technology, which has found usage in applications varying from custom prosthetics to surgical procedures. And with the US Food and Drug Administration (FDA) approving the sale of Spritam (in 2016 across USA), a drug used in preventing seizures, produced by Aprecia Pharmaceuticals using 3D printing, this commercial use of 3D printing technology embodies a momentous development in the field of printing drugs. The deployment of this technology offers certain benefits, but also comes at a cost, and affects the cost dynamics of producing a drug.
Cost savings offered by 3D printing technology are massive. Making drugs using printers will gradually reduce the processing equipment required, allowing the final product to be printed on one versatile machine, saving thousands of dollars. Going a step ahead, pharma companies will provide the base products for printing of the medicines at clinics and pharmacies, which means that the investment in production and storage facilities at the pharma company’s end will decline as the physical making of the drug will be shifted closer to the end-user. The technology will also help save on packaging and labelling costs along with bringing down logistics expenses.
However, as 3D printing capabilities develop further and as the cost of printing drugs falls, increasing easy accessibility to these drugs, it will become imperative to address safety and regulatory concerns associated with this technology.
While making drugs with 3D printing technology could be a game changer for the medical industry, it also comes with a potential threat of counterfeit and illegal drugs. As drugs production will be shifted from centralized location of pharma companies, which are able to ensure more controlled and supervised production processes, drugs will be printed at numerous clinics and pharmacies, and hence strict regulations need to be adopted and methods of production need to be appropriately controlled. Unified safety procedures and quality control measures need to be developed so that patients can be assured of the quality of the products.
The immense potential offered by this technology is increasingly materializing through commercialization in developed markets. However, as massive financial inputs from pharma companies paired with research grants and support by governments are still required, it is fair to believe that this technology is still far out from the reach of the less developed parts of the world, at least in the foreseeable future.